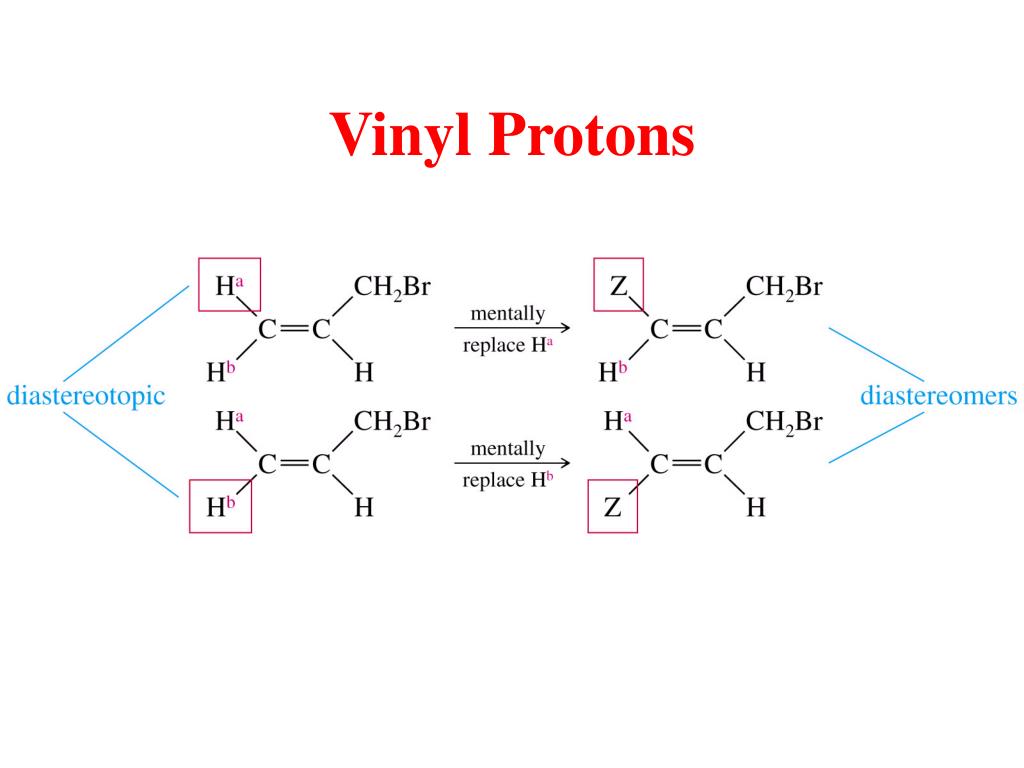
State the approximate chemical shift δ for the following types of protons.
Chemical shift of vinylic protons.
Characteristic proton chemical shiftstype of protonstructurechemical shift ppmcyclopropanec3h60 2primaryr ch30 9secondaryr2 ch21 3tertiaryr3 c h1 5vinylicc c h4 6 5 9acetylenictriple proton nmr chemical shifts california state university stanislaus.
The electrons are said to shield the proton from the applied magnetic field and this means that less energy is necessary to excite the proton from one spin state to another and therefore its chemical shift occurs at lower frequency than it would otherwise.
And even though the signal can be in the range from 1 6 ppm it is usually in the downfield end of this spectrum.
We know that a proton alpha to a carbonyl group is pulled downfield.
Diamagnetic anisotropy is also responsible for the downfield chemical shifts of vinylic protons and aldehyde protons 4 5 6 5 ppm and 9 10 ppm respectively.
Table of characteristic proton nmr shifts type of proton type of compound chemical shift range ppm rch 3 1 aliphatic 0 9 r 2 ch 2 2 aliphatic 1 3 r 3 ch 3 aliphatic 1 5 c c h vinylic 4 6 5 9 c c h vinylic conjugated 5 5 7 5 c c h acetylenic 2 3 ar h aromatic 6 8 5 ar c h benzylic 2 2 3 c c ch 3 allylic 1 7 hc f fluorides 4 4 5 hc cl chlorides 3 4.
Those bonded to carbons which are next to unsaturated centres.
However this particular orientation makes such a large contribution that it dominates the chemical shift.
It is important to understand trend of chemical shift in terms of nmr interpretation.
This is not surprising since the proton is not only vinylic but it is also alpha to a carbonyl group.
Notice that the proton closest to the carbonyl group is at a higher chemical shift than the proton in cyclohexene 6 05 ppm for cyclohexenone vs.
Electronegative groups move to the down field left.
The chemical shift of a vinylic proton is an average over all orientations of the molecule.
The proton nmr chemical shift is affect by nearness to electronegative atoms o n halogen and unsaturated groups c c c o aromatic.
Table of characteristic proton nmr chemical shifts.
Type of proton type of compound chemical shift range ppm rc h 3 1 aliphatic 0 9 r 2 c h 2 2 aliphatic 1 3 r 3 c h 3 aliphatic 1 5 c c h vinylic 4 6 5 9 c c h vinylic conjugated 5 5 7 5 c.
Those bonded to carbon atoms which are in turn bonded to a highly electronegative element.
Splitting between vinylic protons in alkenes depends strongly on the geometrical relation ship of the coupled protons.
C h acetylenic 2 3 ar h aromatic 6 8 5 ar c h benzylic 2 2 3 c c c h 3 allylic 1 7 h c f fluorides 4 4 5 h.